Why the Axolotl Pancreas Could Transform Diabetes Research
The Mexican axolotl is famous for regrowing lost limbs, hearts, even parts of its brain. But until recently, we knew almost nothing about its pancreas—the organ that digests food and keeps our blood sugar in check. A new study steps into that gap, revealing how the axolotl pancreas develops, functions, and even how it responds when a key gene goes missing.
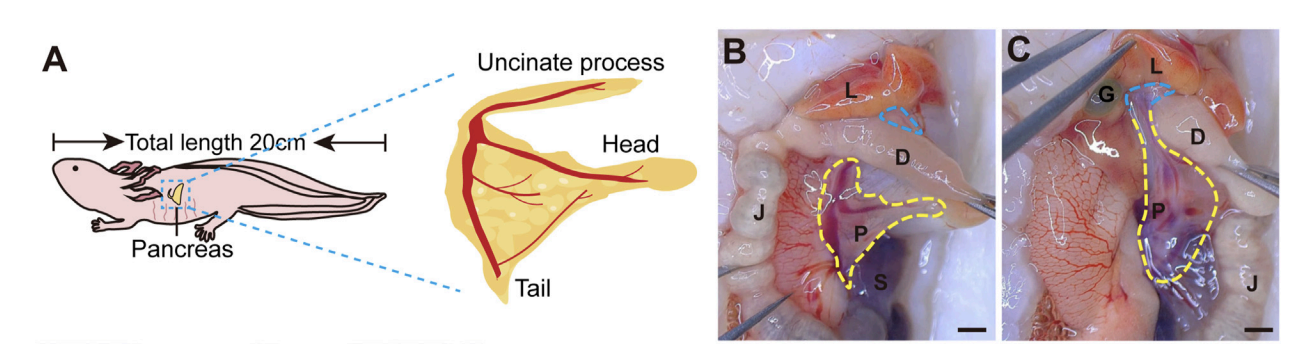
FIGURE 1 The structure of adult axolotl pancreas. (A) The pancreas model of an adult axolotl shows that the basic structure of the pancreas includes the head, body, tail, and uncinate process. (B) Anatomical structure of the ventral view of the pancreas. The pancreas head connects to the duodenum, the tail connects to the spleen, and the body is suspended from the jejunum through the mesentery. L, liver; D, duodenum; J, jejunum; P, pancreas; S, spleen; G, gallbladder. (C) Anatomy of the dorsal view of the pancreas. After turning the jejunum to the right, the uncinate process connects to the liver and gallbladder (blue line).
A salamander with surprising pancreatic potential
Unlike mice or zebrafish—our usual lab stand-ins for diabetes studies—the axolotl sits at an evolutionary crossroads. Its pancreas offers a unique window into how these organs evolved across tetrapods. By mapping cell types and tracking gene activity from larva to adult, researchers discovered the axolotl has all five mammalian islet cell types, plus a few amphibian quirks.
Mapping the axolotl pancreas
The gland is split into head, body, tail, and uncinate process—just like in mammals.
Exocrine cells (ducts and enzyme-makers) and endocrine islets (hormone-secreters) weave through the tissue.
In a twist, cells that in humans only release pancreatic polypeptide also co-make glucagon, suggesting a dual role in digestion and sugar control.
(D) Hematoxylin-Eosin staining of the axolotl pancreas. A, acinar; I, islet; D, ductal; E, erythrocyte; CA, centroacinar. (E–J) In situ hybridization and immunofluorescence staining show the molecular features of the axolotl pancreas. (E) Fluorescence in situ hybridization for Amylase (Amy, green), and Insulin (Ins, red), combined with DAPI (blue) on pancreas paraffin sections. (F) In situ hybridization for Keratin 19 (Krt19, purple) on pancreas cryosections. (G) Immunofluorescence for INS (red), Glucagon (GCG, green), and Somatostatin (SST, white), combined with DAPI (blue) on pancreas cryosections, showing the islets are scattered throughout the pancreas, and the uncinate process has no INS⁺ β cells. (H) Immunofluorescence of a magnified islet shows that the islet consists of a cluster of INS⁺ β cells (red), surrounded by GCG⁺ α cells (green) and SST⁺ δ cells (white). (I) Fluorescence in situ hybridization for Motilin (Mln, white) and Ins (red) on pancreas cross-cryosections shows a very small number of Mln ⁺ ε cells surrounding the β-cell cluster.
Decoding the genetic switchboard
By sequencing RNA in young larvae versus two-year-old adults, the team found a dramatic shift:
Early pancreas: genes for growth, cell division, and tissue formation are blazing hot.
Mature pancreas: hormone production, secretion, and nutrient response genes turn up, while growth genes quiet down.
Comparing to mouse and human data revealed axolotls dial down rapid glucose-sensing and insulin-release genes—clues to their slower metabolism.
Slow and steady wins the glucose race
Standard glucose and insulin tolerance tests needed tweaking for axolotls’ relaxed pace. Instead of minutes, blood sugar peaks took 12 hours post-glucose injection—and returned to baseline by 36 hours. Insulin cutting blood sugar played out over 12 hours instead of the usual couple. This slow-burn metabolism aligns perfectly with the RNA data.
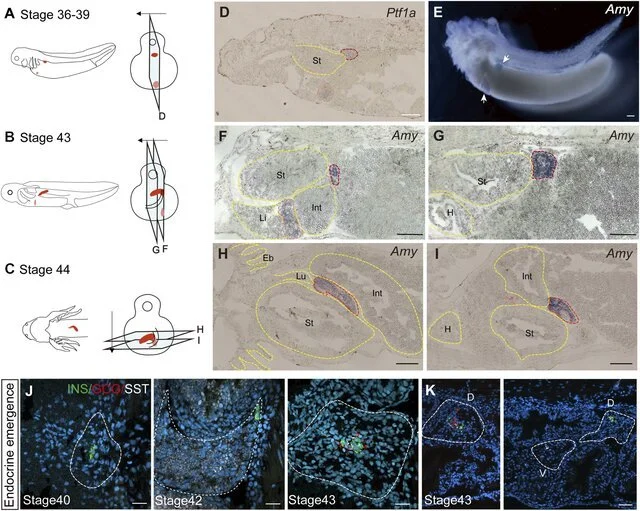
The development of the pancreas in axolotl embryos. (A–C) A model of pancreatic development. At stage 36, the dorsal bud and ventral bud merge. At stage 43, the dorsal bud elongates along intestinal tube. After 5 days, the dorsal bud and ventral bud fuse at stage 44. The arrows indicate the slice direction. The light blue rectangles represent sections shown in panels (D–I). The red section indicates the dorsal bud, and the pink section indicates the ventral bud. (D) In situ hybridization for Pancreas associated transcription factor 1a (Ptf1a, purple) on paraffin sections of embryos shows that Ptf1a is expressed in both the dorsal bud (red circle) and the ventral bud (pink circle) at stage 36. (E) Whole-mount in situ hybridization shows that Amy is expressed in both the dorsal (blue) and ventral (purple) buds at stage 39. (F, G) In situ hybridization for Amy (purple) shows that the dorsal bud extends as the intestinal tube grows. (H, I) In situ hybridization for Amy (purple) shows that the dorsal and ventral buds have fused by stage 44. (J) Immunoflu
Piecing together Pdx1’s role
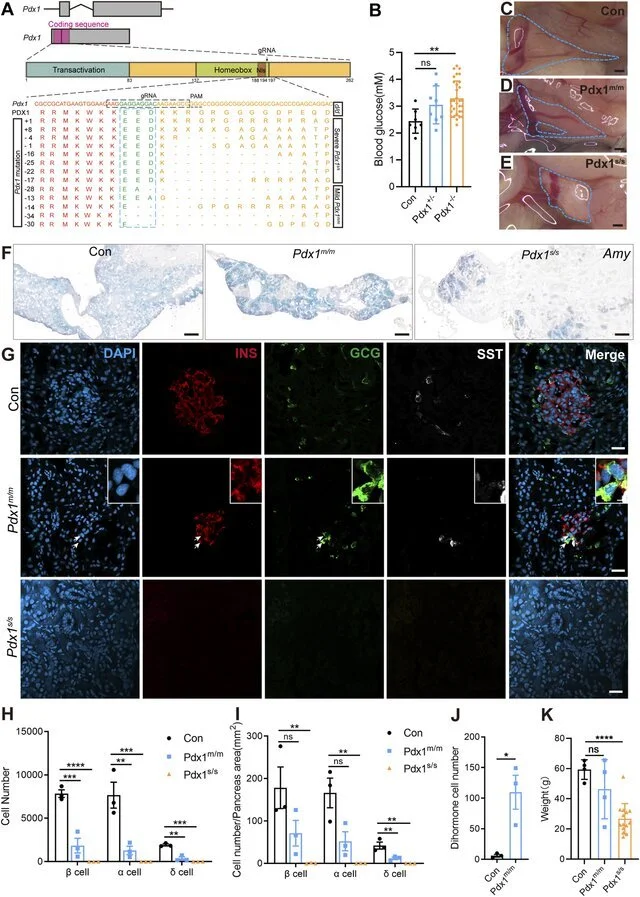
Pdx1 is a master regulator of pancreatic formation in mammals—lose it and you lose the gland. In axolotls, CRISPR-driven Pdx1 knockouts shrank the pancreas, depleted hormone cells, and caused high blood sugar. Yet these mutants survived as dwarfed adults, unlike mice who die shortly after birth. Mild mutants even churned out “dihormonal” cells, hinting at plasticity that could fuel regeneration.
The mutations of Pancreatic and duodenal homeobox 1 (Pdx1) result in varying degrees of absence in both the endocrine and exocrine cells of the axolotl pancreas, attributable to different allele mutations. (A) Schematic diagrams of the Pdx1 gene structure and PDX1 protein domains, along with a schematic illustration of the actual effects of Pdx1 mutations on the proteins. CRISPR gRNAs was designed to target the Pdx1 sequence and injected into one-cell-stage eggs, and resulted in mild and severe phenotypes. Axolotls with a complete homeobox in both Pdx1 alleles (Pdx1 s/s ) exhibit a severe phenotype. In contrast, a mismatch or frameshift mutation in the homeobox region of both Pdx1 alleles (Pdx1 m/m ) results in a milder pancreatic defect. (B) Fasting blood glucose levels in control, heterozygous, and homozygous axolotls show that blood glucose significantly increase in Pdx1 −/− mutants, while no significant change is observed in Pdx1 +/− mutants. (C–E) Anatomical analysis of control (C) and Pdx1 mutant axolotls (blue circle).
What this means for diabetes and beyond
Evolutionary insights: Axolotls bridge gaps between fish and mammals, showing how the pancreas gained new tricks.
Regeneration clues: Their ability to survive with severely damaged glands suggests backup pathways for rebuilding islet cells—a potential goldmine for diabetes therapies.
Metabolic models: A slow-metabolism vertebrate makes it possible to study long-term glucose dynamics in ways impossible in mice or fish.
Stay tuned as we continue to break down the latest research studies involving axolotls!
Source and image credits: https://www.researchgate.net/publication/388969740_Revealing_the_biological_features_of_the_axolotl_pancreas_as_a_new_research_model#fullTextFileContent